
Cobalt compounds have seen heavy use since the early twentieth century, but cobalt isooctanoate really gained ground with the boom in coatings and drying technologies in the second half of the last century. Paint manufacturers scoured for better drying agents as industrial manufacturing picked up pace. Traditional lead-based driers raised health concerns and regulatory hurdles. Cobalt isooctanoate emerged as a more manageable and efficient coordination compound. Chemists tailored its structure for better solubility in organic solvents and improved drying times for alkyd paints. Over time, safety requirements shifted how manufacturers handled cobalt’s toxicity, guiding industry to refine production methods and assess downstream exposure risks. The demand for alternatives and tighter regulations keeps pushing both the chemical synthesis and application fields for this substance.
Cobalt isooctanoate serves as a metal-organic compound mainly in drier systems for coatings, inks, and composite materials. Its key selling point comes from acting as a catalyst for oxidation-induced polymerization in alkyd resins, giving paints, lacquers, and inks their characteristic curing abilities. The compound usually appears as a deep purple or reddish liquid which blends with many common organic solvents. Some variants even show a bluish tint, which speaks to the underlying cobalt content and minor differences in synthesis. Manufacturers often deliver it as a liquid concentrate, sometimes premixed with stabilizers to adjust cobalt levels or improve safety in handling and storage. End-users appreciate its quick kick-start for drying, though they weigh concerns over environmental risks and cobalt’s toxicity.
Cobalt isooctanoate takes the form of an oily, viscous liquid at room temperature. Its color ranges from deep red to violet depending on the concentration and preparation method. The scent can be sharp, with a somewhat metallic undertone. It typically dissolves in aromatic hydrocarbons, mineral spirits, and certain alcohols, making it flexible for blending into various coating formulations. Its cobalt content directly affects reactivity, usually expressed in percent by weight, with common forms running from 6% up to around 12% cobalt. The compound decomposes upon strong heating, releasing fumes that include organic acids and metallic oxides. It reacts with air and moisture over time, though manufacturers stabilize commercial products to resist degradation on the shelf. Its density typically falls near 0.97-1.02 g/cm³, and it resists freezing under most typical storage temperatures encountered in the coatings industry.
Producers provide clear technical data sheets for cobalt isooctanoate, with the cobalt content being a primary attribute. Most products list the actual percentage of metallic cobalt, the solvent base, and the acid number. Buyers also expect to see parameterizations like viscosity, flash point, and specific gravity. Regulations push for proper hazard labeling under GHS standards, spelling out health and environmental risks with clear pictograms, signal words, and response instructions. The label and safety data sheet include UN shipping codes and directions for use, personal protective equipment, waste disposal, and environmental precautions. Certification for REACH compliance or local restrictions appears increasingly often, particularly for shipments into the EU or North America, where traceability and chemical composition face scrutiny.
Manufacturing cobalt isooctanoate usually starts with reacting cobalt(II) salts—commonly cobalt carbonate or cobalt hydroxide—with branched-chain carboxylic acids such as isooctanoic acid. The process often unfolds in a closed reactor under controlled heat and agitation. Excess acid helps drive the reaction to completion and keeps the mixture homogeneous. The resulting product passes through filtration to clear out unreacted solids or byproducts. Cobalt content is tightly monitored throughout the process; small tweaks to temperature or reactant ratios can shift the distribution of cobalt complexes and impact the end-use drying performance. Industrial producers sometimes introduce stabilizers or co-driers to tailor shelf life and compatibility with different coating systems. The solvent base gets selected based on customer demand—whether high-flash for safety or low-odor for specialty coatings.
Cobalt isooctanoate participates in redox and catalytic cycles in organic coatings. The cobalt center shifts between oxidation states as it speeds up peroxide formation and subsequent cross-linking of drying oils and alkyds. Formulators blend it with auxiliary driers—calcium, zirconium, zinc—to avoid unwanted surface wrinkling or yellowing. Cobalt's role can be modified further by changing the ligand; swapping isooctanoate for neodecanoate or naphthenate alters drying rates and compatibility. In harsh conditions, the compound can react with strong acids, releasing volatile organic acids and creating waste management challenges. Test labs experiment with additive packages, adjusting kinetic parameters to minimize environmental loss through volatilization, ensuring the catalyst remains locked into the film as much as possible during curing. Some chemists explore encapsulation or polymer-bound forms to control migration and reduce worker exposure.
Cobalt isooctanoate appears in the market under a handful of chemical aliases. Some buyers may spot names such as cobalt(II) 2-ethylhexanoate, or even cobalt(II) octoate. Specific commercial blends carry trade names crafted by major coating and chemical suppliers, each offering slight tweaks to formulation, drying performance, or safety profile. Producers often cross-list these names on data sheets to meet global distribution needs, reducing purchasing errors or confusion. Alternate spellings, especially for the isooctanoic component, occasionally show up on shipping manifests or in regulatory databases, so careful cross-checking of CAS numbers and detailed chemical descriptions is essential for procurement teams and quality control.
Working with cobalt isooctanoate demands attentiveness to both human and environmental health protocols. Splash hazards and vapor exposure take top priority—aerosol mists or skin contact might sensitize workers or cause rashes. Inhalation can bring acute toxicity, headache, or more severe complications for sensitive individuals. Storage must avoid open flames or strong oxidizers since the product’s organic base and metallic center both present fire and explosion risks in extreme conditions. Facilities handling this substance rely on chemical-resistant gloves, splash goggles, and sometimes respirators, especially when blending larger batches or cleaning up spills. Ventilation and vapor recovery systems in mixing plants help cut worker exposure and fugitive emissions. Regulations press for secondary containment, labeling of containers, and regular training, not only for hazardous waste disposal but also spill response and first aid. Emergency showers and eyewash stations anchor operational best practices in production environments.
Cobalt isooctanoate stands as a backbone material in the coatings industry, defining how fast paints and varnishes cure in architectural, automotive, and industrial settings. Printers use it in inks to control tackiness and set time, especially in high-speed rollers or offset applications where fast throughput matters. Composite makers sometimes employ it to improve resin curing in fiberglass or cast polymer systems. Art conservation attracts a niche use, with certain restoration projects choosing classic drying systems that bring out the original character of historic oil-based paints. Engineers value it for field repairs and touch-up coatings on metals and machinery, relying on its robust curing properties even in changing weather or humidity. In all these sectors, its role links directly to productivity—shorter downtimes, resilient finishes, and lower energy costs, as air-dry systems take less infrastructure than ovens or forced-air lines.
Research in cobalt isooctanoate pivots on three fronts: safer handling, lower toxicity, and greener replacements. Universities and private labs keep probing its molecular interactions in film curing, looking for pathways to cut residual migration and unwanted byproducts. New ligand systems get tested for stability, reactivity, and compatibility with water-borne paint systems, which have grown in popularity for their environmental footprint. Process engineers run pilot reactors with closed-loop solvent recovery or continuous monitoring to spot minor impurities, hoping to lock down the lowest unreacted acid or cobalt traces possible. Collaboration springs up between chemical manufacturers and downstream users, swapping real-world feedback for refinements in formulation. Some work spins out into nanotechnology, exploring how microencapsulated catalysts might offer precise delivery with reduced exposure in plant environments or during application.
Toxicologists and occupational health specialists document the risks of repeated cobalt exposure, with inhalation and dermal absorption presenting the most acute pathways. Industrial surveys draw connections between airborne cobalt concentrations and asthma, dermatitis, or more severe long-term organ effects. Regulatory agencies update recommended exposure limits as more data from animal studies and long-term workplace monitoring comes in. Environmental fate of the compound also triggers concern—cobalt does not degrade quickly in soil or water, risking bioaccumulation and toxicity in various species. Modern risk assessments use advanced analytical techniques like mass spectrometry to trace minute quantities in wastewater or finished coatings. Industry circles keep an eye on global policy discussions about restricting cobalt compounds in consumer products, balancing technical performance against calls for safer alternatives.
Market watchers expect pressure for alternatives to cobalt isooctanoate to intensify, as industries and regulators eye non-toxic or less persistent driers. Bio-derived ligands and hybrid metal complexes draw early researchers, chasing similar performance without the downsides of classic metal-organic driers. Digital process control and in-line quality testing mean users can dial in minimal dosages, trimming waste and exposures. Still, specialty and niche technical markets give this product a lasting, if shrinking, shelf space, especially where performance can't be matched by new entrants. A new wave of innovation may split the difference—retaining some cobalt-based chemistry for high-demand applications, while shifting routine uses to greener alternatives. As research keeps pouring out of labs and environmental rules tighten worldwide, the balance between practical drying needs and health standards will keep evolving.
Cobalt isooctanoate finds its way into more products than you’d think. You won’t see it on store shelves, but it plays a strong supporting role in many industries. In the coatings and paints world, this compound acts as a drier. Whenever I’ve tackled a painting project, from home repairs to helping a friend renovate, waiting for paint to dry feels like the longest part of the job. In industrial settings, waiting isn’t just an annoyance—delays cost money. Cobalt isooctanoate speeds up the drying process of alkyd paints and varnishes. This benefit isn’t just about convenience. Without fast and proper cure times, a new coat wouldn’t set right, leading to a poor finish and sometimes lost revenue.
Cobalt isooctanoate acts as a catalyst. Once it’s in the paint or ink, it helps transform liquid binders with oxygen—from the air—into solid films. I remember my first time learning about paint drying: it’s not just about water evaporating. Oxygen actually reacts with the oil in the paint. Cobalt isooctanoate gives this reaction a push, helping paints cure evenly and quickly. Its use isn’t only in decorative applications. Protective coatings for metal structures, pipelines, and even automotive parts rely on its ability to create durable surfaces that last through weather and wear.
Beyond paints, cobalt isooctanoate has a place in plastic and rubber production. Many plastic parts and rubber items need curing, too. Curing in these industries means building resilient, flexible, and long-lasting products. Cobalt isooctanoate shows up in certain adhesives and sealants, making sure they set right and last long. I’ve noticed that poorly cured rubber loses flexibility and can fail under stress, all because the right catalyst didn’t finish the job.
Cobalt compounds carry health risks if handled carelessly. Cobalt can irritate the skin, eyes, and respiratory tract, and long-term exposure might create more serious health challenges. In jobs I’ve worked that involved industrial paints, safe handling wasn’t up for debate. Anyone working with cobalt isooctanoate needs solid safety training, protective clothing, and good ventilation in their workspace. Companies have to limit exposure and follow regulations, like those from OSHA or the European Chemicals Agency. Environmental release, even in small amounts, poses issues, as cobalt compounds do not break down easily in nature. Spills and improper disposal threaten soil and water.
Despite the compound’s important role, industries are searching for alternatives. Green chemistry trends push for less toxic and more sustainable options. I’ve seen research partnerships between paint companies and universities aimed at creating new catalysts from plant-based or less hazardous sources. But a replacement must do just as good a job speeding up drying without sacrificing finish quality or durability. Change isn’t instant. The right kind of innovation requires investment, testing, and regulation, but pressure to reduce health and environmental risks is growing.
Cobalt isooctanoate will remain important in industrial processes as long as it brings efficiency and quality. Still, every player in the supply chain must balance results with responsibility. That means regular safety updates, environmental checks, and a focus on smarter, cleaner options for the future. Whether you’re painting a wall or producing car parts, understanding what’s in your materials and how they are managed matters more each day.
Most folks in the coatings and paint industry will recognize cobalt isooctanoate thanks to its use as a drier. If you spend any time on production floors or in labs, you’ll spot this cobalt salt as a dark violet, nearly black liquid with a sharp, distinctive aroma. Right away, folks learn there’s a lot of chatter about “the grade” or “metal content”. It’s not fluff. It’s about function and compliance.
You’ll mostly bump into cobalt isooctanoate supplied at 6% metal grade by weight. Every liter from legitimate suppliers lands in that ballpark, give or take a few tenths of a percent. Technical data sheets and safety information nail this down reliably. This isn't just tradition or habit—concentration dictates how much drier ends up in a mix, which affects drying time and color, not to mention potential toxicity and environmental performance.
The industry standard didn’t come from thin air. Over decades of use and testing in paints, alkyds, and inks, a 6% grade provides a sweet spot of active metal content vs. formulation flexibility. Less than that, and manufacturers wind up adding too much liquid, risking trouble with viscosity and solvent thresholds. Too strong, and suddenly you’re dealing with hazardous handling rules, unstable blends, and uneven results in large-scale runs.
If you think back to chemistry classes or have worked on an R&D bench, you'll recall metals as driers act as catalysts. That means the actual percentage affects the whole system's kick-off timing. Push the concentration higher than 8%, and the drier starts to behave unpredictably—there’s risk of gelling the paint or forming unsightly skins. Anything less than 4% takes too long to dry, often failing tight manufacturing schedules.
Cobalt, for all its utility, comes bundled with health and environmental issues. Leading agencies—including the US EPA and the European Chemicals Agency—classify certain cobalt compounds as possible carcinogens. The 6% grade keeps handling safer and helps companies toe the line for workplace exposure limits. I remember the first time I had to complete a Material Safety Data Sheet; the percentage had immediate impact on our mandatory warnings and storage guidance.
The EU REACH regulation pins down use limits for cobalt content in consumer products. Go above published ranges and the paperwork multiplies, audits increase, and product processing slows. Many chemists working in sustainable coatings have said they stick to 6% not just due to performance—but as a move to avoid regulatory headaches and push toward safer practices.
Some manufacturers look for ways to cut cobalt altogether. Manganese and iron-based salts see trials as less toxic, more sustainable alternatives, and these can slip into existing formulas, but the results still fall short on the snap-dry action offered by cobalt. Widening adoption of water-based or UV-cured coatings drops the need for metal driers in the first place, reducing demand for such concentrates.
Companies can train their chemists to blend lower-percentage driers more skillfully, fine-tune solvent content, and watch for storage conditions. A more transparent supply chain, routine third-party lab checks, and clear communication with painters provide practical stops against batch-to-batch surprises or regulatory slip-ups. It’s an ongoing process, not a finished chapter in chemistry.
Professionals, especially newcomers, need to know that even tiny shifts in percentage change their results, expenses, and safety profile. For anyone mixing color in a backroom shop or managing a global materials list, accuracy on the percentage isn’t just bean counting. It shapes product quality, shapes regulatory standing, and, when handled right, supports trust between buyers and developers. Cobalt isooctanoate at 6% might sound like trivia until you see what happens when the number slides, even a little.
Cobalt isooctanoate shows up in places you might not expect. Paints, inks, adhesives, and rubber products can include this chemical. Manufacturers rely on it as a drier to speed up hardening and curing processes. Its use in industry seems ordinary—until questions about safety start popping up. People often handle it without realizing it carries real risks.
You learn some lessons by working in environments where people handle additives like cobalt compounds. Workers sometimes skip gloves or masks, assuming minimal danger. That confidence can be misplaced. Cobalt compounds aren’t exactly benign. Cobalt isooctanoate, for example, can irritate skin, eyes, and the respiratory tract. Repeated exposure, even at low levels, delivers long-term harm. Inhaling powders, mist, or vapors raises concerns for asthma and a type of skin allergy called contact dermatitis. Safety data sheets published by reputable suppliers highlight these effects, based on years of health surveillance in factories.
The conversation gets more serious when talking about what happens inside your body. Cobalt, as a metal, is an essential nutrient in tiny amounts. But in concentrated or repeated doses, it can hurt the thyroid, lungs, or even the heart. The International Agency for Research on Cancer lists several cobalt compounds as possible carcinogens. Workers in industries that use cobalt driers report increased cases of asthma and eczema—these aren’t just statistics; they show up in real families. There have been studies connecting cobalt exposure with problems like “hard metal lung disease.” Not everyone in the industry faces the same levels of risk, but ignoring them never pays off.
Companies don’t always share details about the formulas they use. That lack of transparency can leave people exposed. Someone cleaning up a paint spill might not know they’re touching a hazardous material. Even where regulations require protective gear and good ventilation, smaller workshops and independent contractors cut corners. Sometimes, people don’t get enough information, so they treat these chemicals like household cleaners instead of potential toxins. Information from the European Chemicals Agency and the U.S. National Institute for Occupational Safety and Health backs up that concern: old habits and lack of training leave gaps in safety.
I have seen big improvements when workplaces commit to real safety cultures. Simple reforms make a big difference. Easy-to-read safety labels, scaled-up ventilation systems, and regular training turn risky tasks into safer routines. Regular medical checkups spot health issues early. Substituting less hazardous chemicals where possible—especially in schools or home workshops—keeps people out of harm’s way. Larger suppliers benefit from customer feedback demanding less harmful products, and that pressure trickles down the supply chain.
Regulations alone don’t protect people—real results show up when organizations listen to evidence and workers speak up about risks. Trustworthy information gives people power to make safer decisions, whether on a factory floor or in a hobbyist’s garage. The story of cobalt isooctanoate is a reminder: stay informed, insist on clear labeling, and take exposure seriously. That’s the only way to keep this chemical in check without sacrificing health for productivity.
Cobalt isooctanoate crops up in a lot of industrial production, especially around drying agents for paints and inks. In my years around manufacturing settings, I've seen how deceptively ordinary chemicals can pose real health risks when overlooked. If you’ve ever spilled a solvent or knocked over a drum, you know small accidents turn into bigger problems fast.
Getting storage right means thinking about risk before anything else. Cobalt isooctanoate likes dry, well-ventilated conditions. Damp air or heat speeds its breakdown, which nobody wants near expensive inventory or sensitive projects. In a shop I worked, we stacked drums on wooden pallets, away from sunlight or any spot that got hot by midafternoon. We stuck to metal shelving because plastic sometimes weakens under chemical exposure.
Locking things up tight with clear labels kept wandering hands away. Every barrel showed a date for repackaging or inspection. Even the smallest leaks got flagged. My coworker once left a lid loose — two days later, the air in that corner got harsh enough to hurt your nose. Simple closings and regular walks through storage take just minutes, but they make a difference.
Reasonable handling habits always top the list for safety inspectors. Direct contact with cobalt isooctanoate brings skin and eye irritation, and inhaling the vapors or dust won’t do your lungs any favors. So, gloves (nitrile) matter, not latex, since those don’t hold up well. Face shields or goggles stay useful for splash risk. Working with chemicals after a gym session once, sweat pulled some material through my sleeve and I had a rash for a week — a clear lesson that coveralls count for something.
Breathing in particulates scars lungs over time. Fume hoods help, as do simple respirators. Every lab I’ve seen with a working exhaust outlasted those that went cheap. Dry air moves contaminants up and out, making regular airflow a backbone of chemical safety, especially where the risks can build up quietly.
Quick response to spills keeps danger from spreading. Granular sorbents or sand work well — never use sawdust, since that reacts badly with some organic chemicals in cobalt isooctanoate mixtures. Once, a friend thought paper towels would work. The next morning, the dark stain on concrete made clear things soaked through and needed a full clean.
Disposing of cobalt-based waste isn’t a home project. Licensed recycling and disposal firms know how to keep cobalt from polluting water supplies. More than one municipality cracked down after waste facilities dumped paint sludge irresponsibly. Having an up-to-date record of shipments and using UN-rated drums goes a long way with regulators.
A solid safety culture boils down to habits. Labels stay visible, lids go on tight, and space stays clear. Employees trade tips and stories — real accidents from the field always leave an impression better than any manual. I’ve found that management showing up during audits and asking real questions helps more than policies alone. With chemicals like cobalt isooctanoate, safe practice is less about reading the rulebook, and more about living it every shift.
Cobalt Isooctanoate shows up in places you might not expect, especially in coatings, plastics, and sometimes inks. Most people handle this chemical in liquid form, using it for drying paints and accelerating certain reactions. I’ve seen it move through labs, factories, and workshop tables. No matter the setting, nobody ignores its health risks. Breathing in the vapors, touching the liquid, or getting dust in your eyes can leave you with more than just a headache or a rash. The metal itself is toxic, and the organic side chain boosts its ability to sneak into your body. That’s a problem for your lungs and skin, both short-term and long haul.
I once watched an experienced lab manager treat cobalt isooctanoate like it was hot oil—never casual, never sloppy. The right gloves, usually nitrile or butyl, matter a lot since latex breaks down too fast. Eye protection isn’t just a nice idea; a splash to the eye can cause serious pain and damage. One mistake that sticks with me came from a painter who wiped sweat with a glove smeared in the stuff—his skin broke out, and he didn’t feel right for days. A good habit is to wash hands and arms right after the task, not later.
The smell of cobalt isooctanoate tells you right away—don’t get comfortable breathing around it. Proper ventilation, whether it’s a fume hood, localized exhaust, or a well-placed fan, makes all the difference. Years ago, I learned this lesson after spending half a shift in a space that had nothing more than a cracked window. My head pounded, and my throat burned for hours. Later, the company put in powered exhaust and nobody complained of headaches after that. Small investments like this can save a lot of health trouble.
I’ve seen too many shops stack chemicals on shaky shelves or leave containers open for convenience. With cobalt isooctanoate, sealing up the bottle after use isn’t just neat—it keeps the fumes inside and cuts down on accidental spills. A spill on the floor can make the workplace risky for everyone. The best setups use metal or polyethylene drums with reliable caps, stored away from open flames or strong oxidizers. Because this chemical feeds fire in ways you don’t expect, a dedicated flammable cabinet really earns its keep.
Experience teaches you not to panic, but to move fast. If cobalt isooctanoate touches your skin, you wash it off with running water—five minutes, not just a quick rinse. If it gets in your eyes, it’s a sprint to the eyewash station. Don’t rub, just flush hard. If you breathe in too much, step outside and get help right away. Every workplace that uses this material should keep clear safety sheets nearby, along with contact info for medical help. Simple plans, drilled by every team member, turn a crisis into something you can manage.
The smartest shops keep training fresh. No one should walk in on day one and guess how to handle a drum of cobalt isooctanoate. Simple checklists at each workbench, yearly refresher training, and having PPE in arm’s reach make safety feel normal, not a chore. Regular audits catch shortcuts before someone pays the price. Science backs up these steps—not just to check a box, but to keep teams healthy for the long haul. That’s what builds trust in places where chemicals mix with daily work.
| Names | |
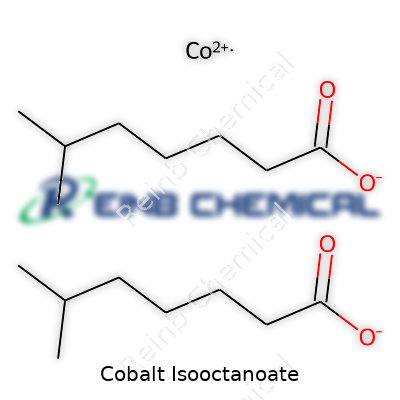
| Preferred IUPAC name | Cobalt(2+) 2-ethylhexanoate |
| Other names |
Isooctanoic acid cobalt salt Cobalt(II) 2-ethylhexanoate Cobalt octoate Cobalt(II) isooctanoate Bis(2-ethylhexanoato)cobalt |
| Pronunciation | /ˈkoʊ.bɔːlt aɪ.soʊˈɒk.tə.neɪt/ |
| Identifiers | |
| CAS Number | 136-52-7 |
| Beilstein Reference | 3958732 |
| ChEBI | CHEBI:86472 |
| ChEMBL | CHEMBL4299619 |
| ChemSpider | 21864786 |
| DrugBank | DB11206 |
| ECHA InfoCard | echa.europa.eu/substance-information/-/substanceinfo/100.109.898 |
| EC Number | 272-773-4 |
| Gmelin Reference | Gmelin Reference: 2093 |
| KEGG | C04252 |
| MeSH | D003041 |
| PubChem CID | 14733278 |
| RTECS number | RH1611000 |
| UNII | 4851976H40 |
| UN number | UN1993 |
| CompTox Dashboard (EPA) | DTXSID60889982 |
| Properties | |
| Chemical formula | C16H30CoO4 |
| Molar mass | 426.454 g/mol |
| Appearance | Clear purple liquid |
| Odor | Odorless |
| Density | 0.95 g/cm³ |
| Solubility in water | insoluble |
| log P | 3.57 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.45 |
| Basicity (pKb) | 7.45 |
| Magnetic susceptibility (χ) | +2400e-6 |
| Refractive index (nD) | 1.480 |
| Viscosity | 12 - 18 mPa.s at 25°C |
| Dipole moment | 2.27 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 723.3 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | V09DX04 |
| Hazards | |
| GHS labelling | GHS02, GHS07, GHS08 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | H317, H319, H334, H341, H350, H360, H372, H411 |
| Precautionary statements | P210, P261, P280, P301+P312, P305+P351+P338, P331, P370+P378, P403+P235 |
| Flash point | > 79 °C |
| Lethal dose or concentration | LD50 (oral, rat): > 2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): >5,000 mg/kg (oral, rat) |
| NIOSH | Not established |
| PEL (Permissible) | PEL: 0.1 mg/m³ |
| REL (Recommended) | 5 – 20 mg Co/kg |
| IDLH (Immediate danger) | 150 mg Co/m³ |
| Related compounds | |
| Related compounds |
Cobalt naphthenate Cobalt stearate Cobalt(II) octoate Cobalt(II) acetate Cobalt(II) 2-ethylhexanoate |